Best Suitable courses for
Life sciences & Pharmacy Graduates
Topics Covered
- Introduction to Clinical Research
- History Behind the development of GCP and Ethical guidelines of Clinical Research
- Glossary terms in CR and ICH GCP Principles
- Drug Development Process
- Stake holders of clinical research and their Responsibilities
- Importance of Monitoring and aUditing in clinical research
- Clinical Trail Design and Its importance
- Essential Documents of Clinical Research
- NDCTA guideline in Clinical Research
- Overview of Clinical Trial Process and Budgeting in Clinical Trials
- Clinical Data Management
- Pharmacovigilance
- Basic Pharmacology
- BA/BE studies
- Drug administration and Drug dosage form
Topics Covered
- Introduction to Clinical Research
- History Behind the development of GCP and Ethical guidelines of Clinical Research
- Glossary terms in CR and ICH GCP principles
- Drug Development Process
- Stake holders of clinical research and their Responsibilities
- Importance of Monitoring and aUditing in clinical research
- Clinical Trail Design and Its importance
- Essential Documents of Clinical Research
- NDCTA guideline in Clinical Research
- Overview of Clinical Trial Process and Budgeting in Clinical Trials
- Clinical Data Management
- Pharmacovigilance
- Anatomy and Physciology(Human Organ System)
- Pharmacology (Drug metabolism, Pharmacokinetics, Pharmacodynamics)
- Routes of Drug Administration, Dosage of Drugs
- BA/BE studies and importance
Key Feature
- Restricted batch strength for better individual attention.
- To train students to achieve an excellent job role in Clinical Research domain
- To enhance and evaluate Clinical practice potential in the field of Clinical Research.
- Opportunity to participate in lively learning environment.
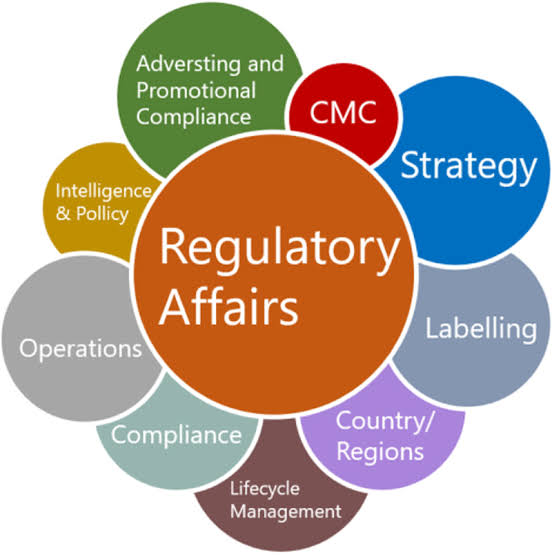
What is Pharmaceutical Regulatory Affairs
The most dynamic job in the pharma and healthcare industry is pharmaceutical regulatory affairs. Generally, pharmaceutical regulatory affairs professionals ensure a company’s compliance with the government’s laws and regulations relating to their business. Each new case comes with a change in old regulations.
The drug development process is largely influenced by the pharmaceutical guidelines that cover quality and safety. Studying drug/pharmaceutical regulatory affairs helps in dealing with these aspects of the pharma or healthcare industry. As a professional in the regulatory affairs area, one can work in drug development industries, food industry, cosmetic industry etc.
In India, admission into pharmaceutical regulatory affairs is provided as a post-graduate program in Pharmacy. However, many organizations are recruiting personnel with M.Sc. (Life sciences, Chemistry) as well as certificate programs in regulatory affairs with RA knowledge.
Course Topics
- 1.ICH Guidelines
- Introduction and General view of Pharmaceutical Industry
- Regulatory Guidelines
- Regulatory Submission
- USFDA
- EMA
- Indian (Domestic)
- Preparing response to 6.Deficiencies
- Biologics guidelines
- Project work
- Resume Preperation as per the Industry requirement and interview Questions
What is Medical Coding?
Medical coding is a process kf transforming or converting the healthcare diagnostic, procedure and other information to Alphanumeric Codes.
Medical cosers help to complete, review, and process medical claims. In the process codes are assigned to medical procedures and diagnoses in a universally accepted medical language.
Medical coding is essential to the healthcare industry as it creates and maintains a single, unified language by which all (physicians, medical administrators, insurance companies, government healthcare officials) integrated parties are able to effectively communicate.
Industry Scope:
Medical Coding
Works in every type of health care facility, Hospitals, Insurance companies, Medical coding (IT) Companies and health care systems.
Medical Coding Designation:
Medical Coding provides a rich career path with opportunities to demonstrate your professional capabilities in an individual contributor role or in a leadership role.
- Jr. Medical Coder
- Sr. Medical Coder
- Quality Analyst (QA/QC)
- Team Lead
- Dy. Manager
- Manager
- Sr. Manager
What is Clinical Data Management?
Clinical Data Management delivers superior-quality, efficient, cost effective solutions assures the trustworthy management and delivery of Clinical Trial study data. Our team manages and best of solutions for our clients that are especially designed according to their needs.
Our experience and understanding of Electronic Data Capture (EDC) processes and systems enables us to provide the optimum solution for each project. Our Data Managers are also trained in the use of the latest standards including CDISC. We employ these standards to enable efficient review, processing transmissions and submission of data to the regulatory authorities. Our proficient project team, is specialize with wide experience & processes in strict compliance to CFR Part 11. Moreover we established the facility, with adherence to sponsor and protocol specific requirements, to complete assignment on time. We employ these standards to enable efficient review, processing transmissions and submission of data to the regulatory authorities.
Clinical Data Management includes the following services:
- Quality Control and Data Quality Audits.
- Medical Coding, SAE Data Reconciliation
- Double Data Entry Comparison and Adjudication
- Query Management Database Lock & Export to SAS
- Clinical Data Management Report Database Design & Development
- Data Cleaning
- Edit, Validation & Design check programming
- Data Validation
- Project Specific CDM Plan
- Delivery of Clean Data Files & Documentation
- Project Data Archival & Storage
- Independent Double-Data Entry

India is becoming a hub for Clinical Research, the demand for professionals in this field is growing rapidly. There will soon be a massive demand for Clinical Research professionals, making it an interesting career option with massive growth potential.
Quick Contact
If you have any questions or need help, feel free to contact us
DIRECTOR
HR MANAGER
Address: Rainbow Clinical Research, Beside Sri Super bazar, Nishigandha Apartments, First floor, Nagoba mandir Road, Nagpur
© Rainbow Clinical Research & Management (RCRM), All Rights Reserved