Services
RCRM consists of a dedicated team of experienced professionals with many combined years' of experience within specialized industries on various scale, training, consulting, and management. Our training includes thorough theoretical and quality live onsite technical training. We assist students in getting job placements on successful completion of training.
Students are shortlisted based on the organization’s requirements. To make students job-ready, RCRM also conducts technical workshops and Industrial Training for Management, Science, Pharmacy, Medical Graduates at college campuses and soft skill seminars. All this helps students find appropriate jobs in the desired industry while also helping save companies’ recruitment costs.
Clinical Research is an organized research on human beings intended to provide adequate information on the drug use as a therapeutic agent on its safety and efficacy. Clinical Research is the application of the scientific method to human health.
Scope of Clinical Research
The roots of clinical Research are wide spread into various fields and the opportunities are wide spread.
Clinical Trial Designation:
- Clinical Research Coordinator/Assistant
- Clinical Research Associate
- Investigator-Sub-investigator, Co-investigator
- Data analyst– Data Manager
- Project Manager
- Medical Writer
- Quality Assurance/Quality Control


Pharmacovigilance begins at the clinical stage and continues throughout the life cycle of the drug.
The process of collection of such information about a drug begins in phase I of the clinical trial, before approval of the drug, and continues even after approval several post-market safety surveillance.
Pharmacovigilance, also known as drug safety, plays a vitally important role in assessing the risk/benefit of pharmaceutical products and ensuring that they are safe for use in patients.
Industry Scope Pharmacovigilance
Good options in Pharmaceutical Companies (MNCs and Indian), Biotech companies, Clinical Research Organizations like Quintiles and Accenture. Regulatory Agencies like DCG (I) & CDSCO. Pharmacovigilance units in Medical colleges and hospitals.
- Trainee Safety Data Analyst
- Junior Safety Data Analyst
- Senior Data Analyst
- Project Coordinator
- Team Lead/Subject Matter Expert
- Assistant Manager
- Manager
- Project Manager
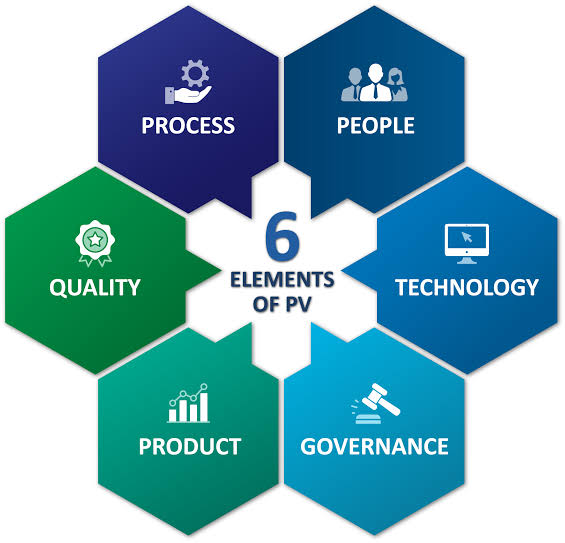

India has developed into a super power for IT skills and has become a major hub for pharmaceutical and biotech manufacturing and contract research.
The outsourcing business in various other segments is also exhibiting promise. Clinical trial data management and statistical analysis is one such area which is growing rapidly, accompanied by a variety of players entering into different models of this business.
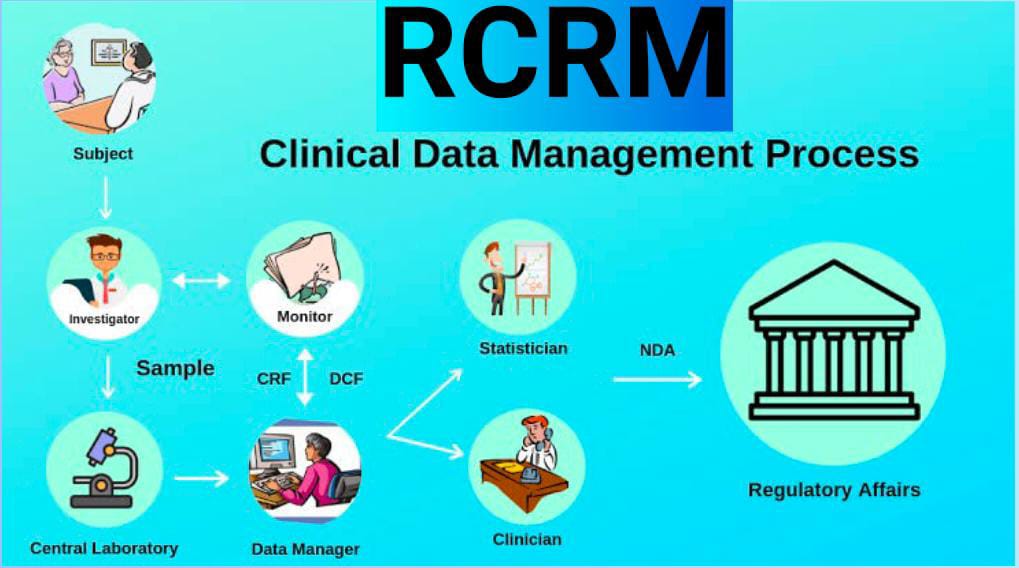

India is becoming a hub for Clinical Research, the demand for professionals in this field is growing rapidly. There will soon be a massive demand for Clinical Research professionals, making it an interesting career option with massive growth potential.
Quick Contact
If you have any questions or need help, feel free to contact us
DIRECTOR
HR MANAGER
Address: Rainbow Clinical Research, Beside Sri Super bazar, Nishigandha Apartments, First floor, Nagoba mandir Road, Nagpur
© Rainbow Clinical Research & Management (RCRM), All Rights Reserved